Consumers’ Medication Schema for Package Inserts
This journal was supported by National Research Foundation of Korea Grant Funded by the Korean Government(MEST)
이 학술지는 2013년도 정부재원(교육과학기술부)으로 한국연구재단의 지원을 받아 출판되었음
Background Due to the recent passage of the over-the-counter (OTC) drug sales bill, thirteen kinds of OTC drugs are now available at supermarkets and convenience stores in Korea. The present study was motivated by this change in OTC drug sales in Korea. To help consumers take OTC drugs appropriately, we investigated how consumers perceived package inserts by investigating their medication schema and further aimed to suggest ways to improve the instruction design of package inserts.
Method We sampled package inserts from the ten best selling OTC drugs in Korea and selected twelve items for the survey. In the questionnaire, the participants were first asked to order the twelve items according to their importance (order task) and then to categorize the twelve items into groups (sort task). In addition to the main tasks, the participants were asked to indicate the items they did not know the meaning of and the unincluded items they wished to have included. Further, they were asked how often they read package inserts. Finally, questions regarding sex and age were asked.
Results To extract the schema, hierarchical cluster analyses were carried out on the data from the order and sort tasks. The results suggest two-cluster solutions for both the order and the sort tasks. For the order task, the three most important items: efficacy & effects, warning, and directions and doses were grouped together and the remaining items were grouped into one group as well. For the sort task, one group included information on medication intake and specific details regarding the drug, such as efficacy & effects, warnings, directions & doses, letter of “misuse/abuse concerns,” names and quantity of active ingredients, methods of storage, and so on. This information is usually what consumers need about medication. The other group included general information about drug and pharmaceutical companies, such as the product name, company name and address, and package unit.
Conclusion The results of the present study provide a possible information design reflecting medication schema and thereby helping people easily access functional information. Imposing structure on package inserts may encourage people to consult them even when there is much information. In addition to structuring, increasing the visibility or salience of important information can enhance its accessibility. Other important improvements to be made are employing easy and frequently used words instead of jargon and difficult Sino-Korean words, and avoiding overly technical and detailed information.
Keywords:
Medication schema, Self-medication, Package inserts, Over-the-counter drug1. Introduction
In May 2012, the over-the-counter (OTC) drug sales bill was finally passed in Korea. Until then,only pharmacists could sell non-prescription drugs in Korea. Due to the passage of the bill, twenty kinds of OTC drugs are now available at supermarkets and convenience stores, which makes well-informed ‘self-medication’ more critical. In many developed countries, self-medication is now common and people independently make decisions regarding their health. Self-medication has merits in that it promotes consumer involvement in managing their health, increases patients' choices and reduces governmental costs in healthcare (Berry, Raynor, Knapp & Bersellini, 2004).
However, risks are associated as well. Consumers often have unwarranted assumptions about OTC drugs, such that they are safe and therefore do not cause any real harm to their health due to their easy access (Clark, Layton & Shakir, 2001; Roumie & Griffin, 2004). Hence, medication information that consumers actually read and understand should be available so that they can use the drugs safely and effectively.
To accomplish this, we need to understand which information is important to consumers in medication and how to structure the instruction information and to reflect this understanding into the design of package inserts.
The present study was motivated by this change in OTC drug sales in Korea. To help consumers take OTC drugs appropriately, we investigated how consumers perceived package inserts by investigating their medication schema and further aimed to suggest ways to improve the instruction design of package inserts by considering the schema.
1.1. Package inserts in Korea
Package inserts are one of the important sources for drug information, where consumers or patients can obtain such information on their own. However, the readability and comprehensibility of the inserts printed in Korea are in doubt. The report by the Consumer Safety Center (2008) in Korea revealed that 99.2% out of 123 leaflets examined used a font size smaller than 8 point, which is the recommended font by the guideline of KFDA (Korea Food & Drug Administration); moreover, 66.1% used a font size smaller than 6 point and 91.8% used more than one difficult technical term. In addition, the dosage instructions were occasionally given inappropriately. In some drugs, the recommended dosage was expressed in ‘mg’ even when the drugs were in the form of a tablet or a capsule, which makes it difficult for consumers to take the exact amount. Problems of how Korean consumers perceived the package inserts surfaced through this investigation; small font size, difficult terms and texts leveled at medical specialists were mentioned as the primary issues (Song, Lee, Kim & Lee, 2008).
As Figure 1 shows, a large amount of content is written in small font and moreover, the items are not structured based on item importance or frequency of use, engendering low readability and accessibility. In another study, consumer competency of information on medication was examined by using ‘the label reading survey’, which was adjusted to fit Korean consumers (Marietta, Welshimer & Anderson, 1999; Nam & Yoo, 2010). Information on the three best selling OTC drugs, analgesic, antacid and anti-diarrhea, was tested. One thousand consumers participated in the survey and the results revealed that respondents correctly answered 3.82 questions, on average, out of 10 questions, which indicates an overall low knowledge level.

An illustration of a package insert and packages printed in Korea (http://blog.daum.net/moge-family/534)
The guideline by KFDA on how to present the information of drugs in containers, packages and package inserts suggests eighteen items and their presentation order (Table 1). Compared to the other countries, eighteen items are a high figure. For example, the U.S. FDA suggested eight items, such as active ingredients, purposes, uses, warnings, directions, other information, inactive ingredients and questions. The FDA recommends companies to provide the drug facts in the listed order and to be easily read and understood. In England, five items, such as name of the medicine, strength, route of administration, posology and warnings, are regarded as the most vital drug facts. They need be presented in the same page without the interruption of logo or graphics and should be printed in a font as large as possible; further, there should be enough space between letters and lines for readability.
In addition to the large number of items, texts are written in a small font size and are tightly spaced, which discourages consumers from reading the inserts. There are difficult Sino-Korean1 words and the suggested order does not reflect the importance of information with regards to medication and the frequency consumers consult the information. The most important and useful information, such as efficacy and effects, warnings, directions and doses, were usually provided in the middle of package inserts without any emphasis or highlights (Song et al., 2008). There must be reasons as to why such a great deal of information is provided. Possible reasons may be protecting consumer rights to know the information, preventing consumers from misusing or abusing drugs by providing enough information, or defending manufacturers or sellers against the responsibility for misuse or abuse. However, too much information is not useful and only reduces the readability and comprehensibility if it is not organized in a way such that consumers can easily process the information.
1.2. Medication schema
In cognitive psychology, there is a concept called schema, which refers to how knowledge is represented in our memory. As experiences of objects, events, situations or actions are repeated, some generalization or abstraction occurs and a mental structure called schema is formed (Rumelhart & Ortony, 1977). Schema helps us understand, memorize and make inferences, allowing for efficient information processing. There can be various kinds of schema; according to Morrow and colleagues, people come to structure their medication knowledge through repeated medication experiences and this organized knowledge structure is called as medication schema. The instructions compatible with their schema help people understand and remember medication information and thereby increase adherence (Morrow, Leirer, Altieri & Tanke, 1991; Morrow, Leirer, Andrassy, Tanke & Stine-Morrow, 1996).
Morrow et al. (1991) examined medical instructions and asked older adults to indicate how 10 items should be ordered in the instructions. The ten items were identified as important factors in a previous study (Morrow, Leirer & Sheikh, 1988). The results revealed that the general schema was shared. The items were grouped into three categories: general information (doctor name, medication name and purpose), intake (dose, schedule, duration and warnings) and possible outcomes (mild and severe side effects, emergency information). In another study, they examined younger and older adults and demonstrated that they shared a similar schema with the 10 items, which were divided into two groups: description of medication and intake, and the potential problems associated with taking the medicine and what to do if complications occur (Morrow et al., 1996). They first demonstrated the medication schema and then conveyed that participants recalled medication instructions better when the instructions were designed to be compatible with their schema.
Maat and Lentz (2010) also demonstrated that when information leaflets were structured according to readers' pre-existing schema, accessing and comprehending the information improved. When people read functional or informational text, they do not simply read it from the beginning to the end. Instead, they navigate through it by skimming, searching and re-reading it (Hartley, 2004; Wright, 1999). Therefore, it is important to structure the text in a way that people can easily access information that they want to read. In order to enhance accessibility, they changed the structure of the side effects, which was grouped by organ systems, to the structure grouped in terms of frequency of information use and severity of the effects.
Similarly, Gray, Cantrill and Noyce (2002) suggested a concept called ‘health repertories’. It refers to the schema people have for selfmedication of minor ailments. Based on the data from focus group interviews, structured interviews and depth interviews of young adults in the U.K., they demonstrated that young adults developed schema for the management of minor ailments. The schema included description/ labeling of symptoms, one or more self-medication strategies and contingency plans in case the strategies failed. Gray et al. (2002) also suggested that this schema is developed through a consumer socialization process and personal illness episodes, and it is dynamic because it keeps changing with new experiences.
The previous studies clearly show that Korean package inserts are not user friendly both perceptually and cognitively. Based on the research on medication schema, the present study aims to investigate the medication schema that Korean consumers have regarding package inserts and to suggest ways to design instruction information so that the accessibility and utilization of the package inserts can be improved.
2. Methods
2.1. Participants
One hundred forty five undergraduate students participated in this study (male: 58, female: 79, no answer: 8). They were from two universities in Seoul. Their mean age was 27.52 (SD=10.24).
2.2. Procedure and questionnaire
Questionnaires were distributed at the beginning of the class and students filled them out for approximately ten minutes. For the questionnaire, we sampled package inserts from the ten best selling OTC drugs in Korea. Despite the KFDA guideline, we wanted to gather the medication information presented to the consumers in the market. From this sampling, twelve items were gathered and they were presented in the questionnaire (Table 2). We did not include the letter of “over-the-counter drug” because all of the sampled package inserts showed this letter either at the right or left heading separately from the other content.
Based on the study by Morrow et al. (1996), the participants were first asked to order the twelve items according to their importance (order task) and then to categorize the twelve items into groups (sort task). They could make any number of groups as they thought necessary. In addition to the main tasks, the participants were asked to indicate the items they did not know the meaning of and the unincluded items they wished to have included. Further, they were asked as to how often they read package inserts; five options were provided: (1) never read, (2) sometimes read, (3) often read, (4) always read, or (5) read for the first time. Finally, questions regarding sex and age were asked.
2.3. Statistical analysis
To extract the schema, hierarchical cluster analyses were carried out on the data from the order and sort tasks. For the order task, a 12×12 matrix with a distance between every pair of items was created for each participant, and the mean distance matrix across the participants was analyzed. For the sort task, a 12×12 matrix with the number of participants who put each pair of items into the same group was created and analyzed. Because the order and the sort tasks provide different information, different matrices were created. A distance matrix was created for the order task, while a frequency matrix was created for the sort task (Morrow et al., 1996). In the hierarchical cluster analysis, Ward’s method was used, which is known as more informative than other methods because it is less influenced by the overlapping clusters (Aldendorfer & Blashfield, 1984).
For the data analysis, SPSS 12.0 was used. For the other questions, descriptive statistics were reported.
3. Results
3.1. Descriptive statistics
Regarding the frequency of reading package inserts, the results indicated that 35.2% of the respondents read them sometimes, 22.8% of them read them only at the first time, and 9.7% of them never read it. These results indicate that half of them do not often consult thepackage inserts when they take medicine (Table 3).
The results of the order task are presented in Table 2. Information related medicine intake (effects, warnings & dosage) turned out as important, whereas details about drugs, such as shape, package unit and name of tar color, turned out to be less important. After the order task, we asked the participants to write down the items that they did not know the exact meaning of. Some participants listed more than one item; in total, one hundred fifty six items were mentioned as being difficult to understand. ‘Description of drugs’ was the most often mentioned item (Table 4).
The reason that this item was chosen seems to be attributed to the fact that the words are in Sino-Korean. Although the Chinese characters of the word are not difficult to comprehend, the Korean word displayed is not an often used one, making it difficult to understand. Furthermore, the names of tar color and preservatives seem to be overly detailed with specialized information.There were thirty eight items as the unincluded items they wished to have included. However, fifteen of them (39.5%) were items already in the package inserts, such as side effects and contraindications. This well demonstrates that people have difficulty in finding the information they need. Fourteen of them (36.8%) were about the alternative presentation of the items already in the package inserts, such as dosage according to age, dosage indication such as prescription drugs (e.g., how many or much to take in the morning, afternoon and night), and easier presentation of the effects and side effects. Only four (10.5%) were new items, such as providing the probability of side effects, daily practices inducing the effects of drugs, how to dispose drugs and symptoms of misuse or abuse.
3.2. Hierarchical cluster analysis
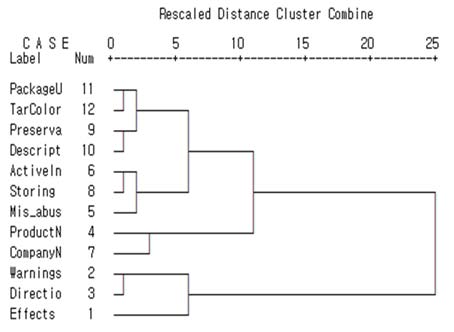
Figure 2 and Table 5 show the graphical and numerical summaries of the cluster solution of the order task. Figure 2 shows that two clusters are formed at the distance of 20~25 and Table 5 also shows that change between coefficients was the largest from stage 10 to 11, which suggests a two-cluster solution. The three most important items, efficacy & effects, warning, and directions and doses, were grouped together and the remaining items were grouped into one group as well.
For the sort task, the average number of categories that the participants created was 3.55 (SD=0.69). Out of 145 participants, eighty eight (60.7%) created four categories, thirty eight (26.2%) created three, nine (6.2%) created two, two (1.4%) created one, and eight (5.5%) did not answer. The results conveyed that the twelve items were divided into two categories.
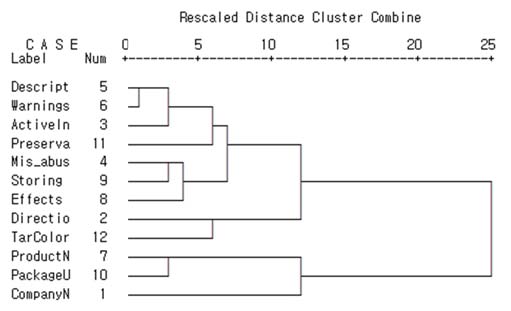
Figure 3 and Table 6 show the graphical and numerical summaries of the cluster solution of the sort task. Figure 3 shows that two clusters are formed at the distance of 20~25 and Table 6 also shows that change between coefficients was the largest from stage 10 to 11, which suggests a two-cluster solution. One group included information on medication intake and specific accounts on the drug, such as efficacy & effects, warnings, directions & doses, letter of “misuse/abuse concerns,” names and quantity of active ingredients, methods of storing, names and quantity of preservatives, description of drugs and the names of tar color. This information is usually what consumers need and use for medication. The other group included general information about drug and pharmaceutical companies, such as the product name, company name and address, and package unit.
4. Conclusion
The results from the hierarchical cluster analysis of the order and the sort tasks suggest that package information can be arranged into two groups, one with information on medication intake and specific accounts on drugs and the other with general information of drugs. Moreover, medication information can be organized according to importance; efficacy and effects, warnings, and directions and doses should be located first and the remaining afterwards. Information order is important not only for accessibility, but also for memory. People often remember well the information at the beginning and the end rather than in the middle, which is referred to as the ‘serial position effect’ (Day, 2006; Postman & Phillips, 1965). Instead of changing the presentation order, emphasizing important information can be another way to improve accessibility. Using a different color or boxing important information can be possible options.
Currently, Korean package inserts are not structured. Information is just listed item by item. KFDA recommends a presentation order of eighteen items; however, the order is not based on an empirical research examining the frequency, importance or schema. Providing only the information is not sufficient for package inserts. The participants of the present study mentioned the items that were already in the package inserts as items to be included, which indicated clearly that people were not able to locate the appropriate and important information. According to Wright (1999), the key processes of reading functional documents, such as package inserts, are accessing, comprehending and applying the information. Therefore, package inserts should be structurally designed in a way to help medical consumers find the relevant information. The results of the present study provide a possible information design reflecting medication schema and thereby helping people easily access functional information. Imposing structure on package inserts may encourage people to consult them even when there is much information. In addition to ordering, using category headers, inserting space between categories, or using systematic spacing can be helpful in organizing the contents. Headers or item titles can be emphasized with boldface, a larger font size or italicized letters. Increasing the visibility or salience of important information can enhance its accessibility.
Other important improvements to be made are employing easy and frequently used words instead of jargon and unfamiliar Sino-Korean words. KFDA listed difficult words and recommends not using them; yet, they are still in use. With easier access to over-the counter drugs, providing easy information for comprehension becomes more critical because consumers decide what and how to take medication without the help of pharmacists. The results of the present study showed that almost half of the respondents did not know the meaning of one item, ‘description of drug’, because it is in Sino-Korean and is not a common word. The word should be replaced with an easy and often used word. Otherwise, people will not even attempt to read it because they do not know what it refers to.
Some items such as name of tar color and preservatives are too technical and detailed. The information itself may be no use to medical consumers and may discourage them to consult the package inserts by giving the impression that the inserts are difficult to understand. Therefore, providing too technical and complex information should be reconsidered and instead, information leveled at general consumers should be provided.
In the present study, we did not redesign a package insert to compare it with the original one in order to examine if the redesigned one enhances the cognitive accessibility of the information. With regards to this, a future empirical research is in need. In addition to the ordering and spatial layout, alternative representations of drug information (e.g., pictogram) can be tested as well for readability and comprehensibility. Further, we extracted schema mostly from young adults. A future study should test similar schema of older adults and observe what kinds of adjustments should be made in order to enhance the readability and comprehensibility of the package inserts for older consumers.
Glossary
1 Sino - Koran refers to the Korean vocabulary that is of Chinese origin or makes use of morphemes of Chinese origin (Wikipedia).
2 Figure Note. PackageU: Package unit, TarColor: Name of tar color, Preserva: Names of preservatives and quantity, Desript : Description of drug, ActiveIn: Names of active ingredients and quantity, Storing: Methods of storing, Mis_abus: letter of “misuse/abuse concerns,” ProductN: Product name , CompanyN: Company name and address, Directio: Directions and doses, Effects: Efficacy & effects.
3 Figure Note. PackageU: Package unit, TarColor: Name of tar color, Preserva: Names of preservatives and quantity, Desript: Description of drug, ActiveIn: Names of active ingredients and quantity, Storing: Methods of storing, Mis_abus: letter of “misuse/abuse concerns,”ProductN: Product name, CompanyN: Company name and address, Directio: Directionand doses, Effects: Efficacy & effects.
Acknowledgments
This study was financially supported by Seoul National University of Science and Technology.
Notes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted educational and non-commercial use, provided the original work is properly cited.
References
- Aldendorfer, M., & Blashfield, R. (1984). Cluster analysis. Newbury Park,CA: Sage.
-
Berry, D., Raynor, T., Knapp, P., & Bersellini, E. (2004). Over the counter medicines and the need for immediate action: a further evaluation of European Commission recommended wordings for communicating risk. Patient Education & Counseling, 53, 129-134.
[https://doi.org/10.1016/S0738-3991(03)00111-3]
- Clark, D., Layton, D., & Shakir, SAW. (2001). Monitoring the safety of over the counter medicines. British Medical Journal, 323, 706-707.
- Consumer Safety Center. (2008). Report on the current state ofindication of over the counter drugs in domestic and foreign market. from http://www.ciss.or.kr:80/jsp/home/common/rss.jsp?jbgbn=0802&rssId=jungbo.
- Day, R. S. (2006). Comprehension of prescription drug information: Overview of aresearch program. Proceedings of the American Association for Artificial Intelligence. from www.aaai.org.
-
Gray, N. J., Cantrill, J. A., & Noyce, P. R. (2002). Health repertories: An understanding of lay management of minor ailment. Patient Education & Counseling, 47, 237-244.
[https://doi.org/10.1016/S0738-3991(01)00226-9]
- Hartley, J. (2004). Designing instructional and informational text. In D. H. Jonassen & P. Driscoll (Eds.), Handbook of Research for Educational Communications and Technology: A Project of the Association for Educational Communications and Technology . Mahwah, NJ: Lawrence Erlbaum Associates, 917-947.
-
Marietta, A. B., Welshimer, K. J., & Anderson, A. L. (1999). Knowledge, attitudes, and behaviors of college students regarding the 1990 nutrition labeling education act food labels. Journal of the American Dietetic Association, 99, 445- 449.
[https://doi.org/10.1016/S0002-8223(99)00108-X]
- Matt, H. P., & Lentz, L. (2010). Improving the usability of patient information leaflets. PatientEducation & Counseling, 80, 113-119.
- Morrow, D. G., Leirer, V., Andrassy, J. M., Tanke, E., & Stine-Morrow, E. (1996). Medication instruction design: Younger and Older adult schemas for taking medication. Human Factors, 38, 556-573.
- Morrow, D. G., Leirer, V., Altieri, P., & Tanke, E. (1991). Elders’ schema for taking medication: Implications for instruction design. Journal of Gerontology: Psychological Sciences, 48, 378-385.
- Morrow, D. G., Leirer, V., & Sheikh, J. (1988). Adherence andmedication instructions: Review and recommendations. Journal of the American Geriatric Society, 36, 1147-1160.
- Nam, S. J., & Yoo, H. J. (2010). Information on medication andconsumer competency. Consumption Culture Study, 13, 21-37.
-
Postman, L., & Phillips, L. W. (1965). Short-term temporal changes in free recall. Quarterly Journal of Experimental Psychology, 17, 132-138.
[https://doi.org/10.1080/17470216508416422]
-
Roumie, C. L., & Griffin, M. R. (2004). Over the counter analgesics in older adults: A call for improved labeling and consumer education. Drug Aging, 21, 485-498.
[https://doi.org/10.2165/00002512-200421080-00001]
- Rumelhart, D. E., & Ortony, A. (1977). The representation of knowledge in memory. In R. C. Anderson, R. J. Spiro, & W. E. Montague (Eds.), Schooling and the acquisition of knowledge . Hillsdale, NJ: Lawrence Erlbaum Associates, 99-135.
- Song, V. K., Lee, J. H., Kim, J. H., & Lee, S. H. (2008). Improvement of marking system and others for preventing medical supply misuse. The Annual Report of KFDA, 12, 643-644.
- Wright, P. (1999). Designing healthcare advice for the public. In F. Durso (Ed.), Handbook of applied cognition . Chichester, UK: JohnWiley & Sons, 695-724.